
The European Commission’s legislative proposal for the deregulation of new GMOs – or new genomic techniques as they are also known – is to be delayed from June 7th 2023 until at least the latter half of that month. Mathieu Willard contextualises recent events surrounding the controversial proposal with an overview of the current GMO regulatory framework and the possible policy options hinted at in an impact assessment leaked by AGRA FACTS.
By Mathieu Willard
In September 2021, the EU Commission published an inception impact assessment, effectively starting the process of deregulating GMOs. The incoming proposal aims to exempt plants derived from certain more recently developed genome modifying techniques from the current GMO legislation. You might know those techniques as “targeted mutagenesis”, “cisgenesis” and “CRISPR”, or, “new genomic techniques” (NGTs) as they’ve been collectively branded by the Commission.
Proposal delayed amid mounting pressure on the Commission
On March 31st, DG SANTE confirmed that the Commission’s proposal on GMO deregulation, as well as the proposal on seed marketing, will be postponed. Originally scheduled for June 7th, it will not be presented before the second half of June or later in July.
As reported by AGRA FACTS [N°28-23], the Regulatory Scrutiny Board, whose role is to ensure the quality of the Commission’s impact assessments, sent the proposal and accompanying Impact Assessment back to the drafting stage. Reasons mentioned are insufficient assessment of the impact on consumer trust, the organic sector, environment and health.
The pressure is increasing on the Commission as, on March 16th, several environment ministers (Germany, Hungary, Luxemburg, Slovenia, Slovakia and Cyprus) stated their support for the position of the Austrian environment minister to maintain the application of scientific risk assessment and mandatory labelling for NGTs. Recently, the German agricultural minister also came out against deregulation of GMOs.
In a previously leaked Farm to Fork document from DG AGRI entitled an “overview of the politically sensitive topics”, the Commission already labelled NGTs as highly sensitive and pointed at general public rejection of the plan. Considering the increasingly politicised nature of the file, it is anticipated that the proposal could be delayed further, until the next Commission’s administration.
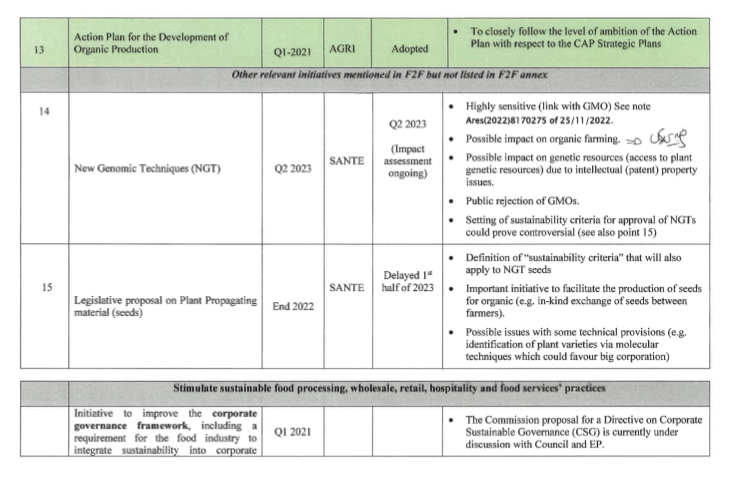
It is important to note that the seed marketing proposal has been delayed as well. This is one more indication that the two proposals are seen as a package. Indeed, one policy option for the upcoming seed marketing revision is the inclusion of sustainability criteria to be met for registration in the EU catalogue. It is foreseen that the criteria will be made to fit GMOs’ characteristics.
Leaked document provides insight on policy options
AGRA FACTS [N°28-23] has shed light on a leaked draft Impact Assessment (IA) summary that was presented to the Regulatory Scrutiny Board. However, before delving into the options outlined in the leaked document, it’s crucial to first review the existing legal framework and context.
-
The current GMO framework
The current GMO regulatory framework is generally considered a balanced piece of legislation, as it doesn’t ban GMOs in the EU but regulates their entry into the market. The framework has three basic principles: prior risk assessment, traceability, and labelling. Directive 2001/18/EC and Regulation 1829/2003 outline the authorization procedures, while Regulation 1830/2003 frames the rules on labelling and traceability.
At the heart of the traceability procedure is the requirement that applicants who intend to introduce GMOs to the EU market must develop and provide a detection method. This obligation enables any user to distinguish GMOs from other organisms not subject to the restrictions and can thus protect farmers from infringement allegations on patented products.
This legislative package is the result of a long and victorious campaign led by citizens and farmers more than two decades ago. The arguments at the time were simple and still hold today.
First, there is a need for caution when products and/or organisms are to be released into the environment. A lack of scientific knowledge about the consequences of such releases should lead to restrictions of use. This need is supported by the precautionary principle, enshrined in the Treaty on the Functioning of the European Union (TFEU), article 191.
Second, consumers also have a right to choose what they want to eat and base their choice on factual information.
Third, farmers should have a right to food sovereignty, to decide which seed they want to use, to a clean and safe environment and to biological diversity. All of which are now part of the United Nations Declaration on the Rights of Peasants and Other People Working in Rural Areas (UNDROP).
The current framework has been pretty restrictive until now, at least when considering direct releases into the environment. As of today, only 8 GMOs are authorised for direct release in the EU (through Directive 2001/18/EC). However, dozens of GM soybeans and maize are allowed to be imported and used as animal feed through Regulation 1829/2003, as well as GM cotton, and end products (such as meat, milk, eggs) do not need to be labelled.
-
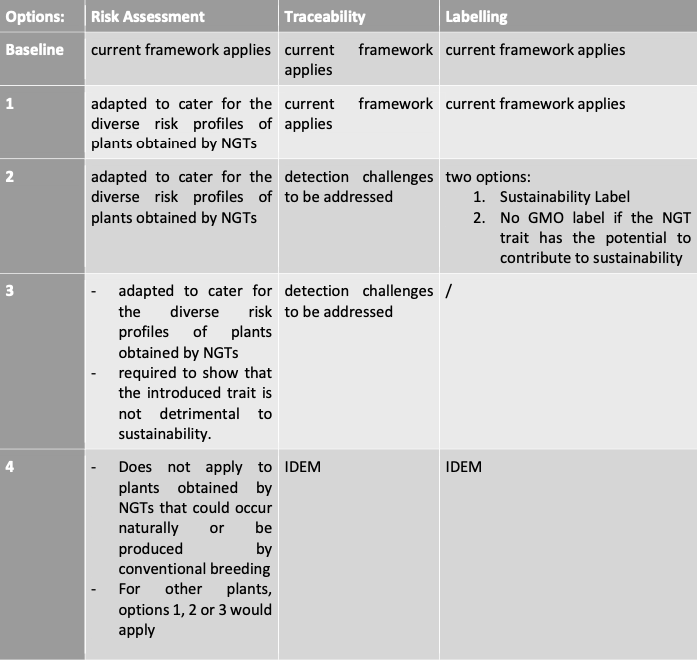
Policy option hints
In the leaked draft IA, five policy options were presented:
What’s next?
Taking the time to clearly define the context is a necessary first step. In the coming weeks, we want to look closely at the Commission’s arguments for deregulation. For example, the Commission seems confident that GMOs produced through NGTs will contribute to the sustainability of our food systems. But what GMOs are actually being developed worldwide?
Opening the door to GMOs would also mean opening the door to patents. What is the Commission’s or the industry’s answer to that issue? What would be the consequences for farmers?
Although the Commission seems to acknowledge that detection challenges need to be addressed, the Regulatory Scrutiny Board specifically noted that there were still issues regarding cohabitation with organic production. What should be done to prevent contamination of organic fields and is it at all preventable?
Stay tuned for future articles.
More on GMOs:
New Genomic Techniques in the EU – on the Road to Deregulation?





1 Trackback / Pingback
Comments are closed.